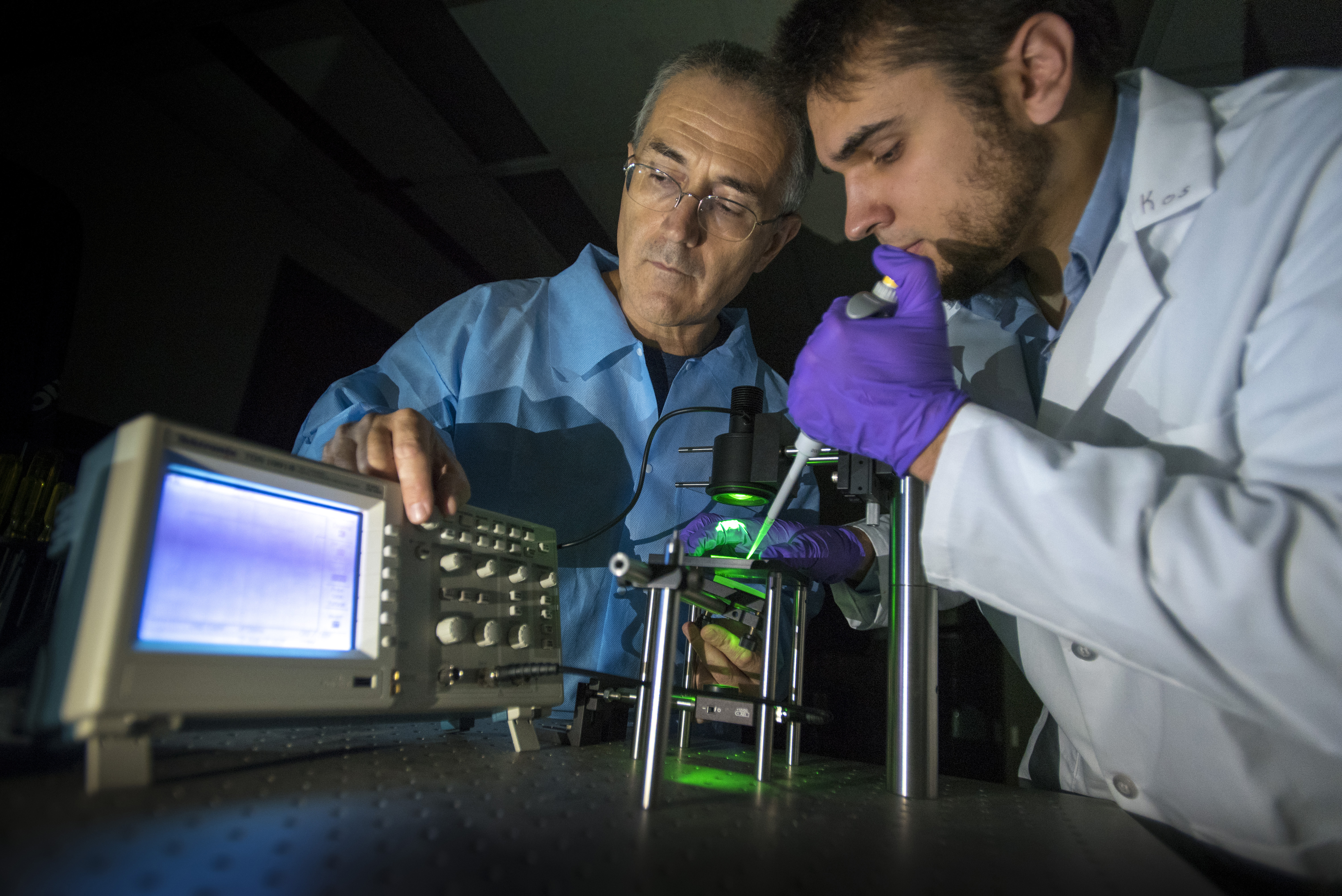
UConn researchers from the Department of Mechanical Engineering have developed a device that tests an important indicator of heart health that is often ignored – blood viscosity.
Blood can be a window into the health of your heart. Doctors are often on the lookout for some common signs that might point to an issue, like abnormal cholesterol levels or high blood pressure. From heart attacks to strokes, routine blood tests can screen for several types of life-threatening cardiac events. But less attention has been paid to blood viscosity.
Viscosity measures a fluid’s resistance to flow. Thick or sticky liquids like honey have high viscosity, while thin, watery liquids have low viscosity. In the case of blood, higher viscosity may signal potential problems, since the heart needs to work harder to pump sticky blood. Thick blood also means organs and tissues receive less oxygen and may cause damage to the lining of blood vessels due to increased friction as blood travels throughout the body.
Studies have shown that increased blood viscosity was significantly more prevalent in patients who experienced heart attacks and strokes compared to patients with lower blood viscosity. In fact, one study found that increased blood viscosity is a more likely sign of a potential cardiac event than high blood pressure, gender, or smoking.
Yet despite this strong correlation, physicians can’t currently evaluate blood viscosity at routine office visits.
“We were very surprised that there is no commercial option to quickly and simply check this critical piece of information,” says associate professor of mechanical engineering and co-inventor, George Lykotrafitis. “The research shows there is a connection between blood viscosity and cardiac events, and the equipment exists to test it, but not in a practical or efficient way. We decided to try to solve the problem.”
So Lykotrafitis and doctoral candidate Kostyantyn Partola developed a small electronic device that can measure blood viscosity at the point of care. The pair recently filed a provisional patent on their invention with the help of UConn’s Technology Commercialization Services.
“Our technology really is plug and play, but the impact is significant,” says Partola. “With this information, doctors can suggest simple life-style changes on the spot to prevent their patients from having a stroke or heart attack.”
Lykotrafitis and Partola’s device may be simple, but the science behind it is specialized and tailored to blood analysis. Blood behaves as a non-Newtonian fluid, which means that its viscosity changes depending on its velocity at any given time. Since the velocity of our blood differs when pumping and at rest, its viscosity also changes. This can be a complication for commercial instruments that are currently used to measure viscosity, but not for the device Lykotrafitis and Partola have developed.
Here’s how it works. A clinician places a droplet of blood onto a small card of transparent plastic containing a microchannel. The blood wicks into the microchannel and flows through a small groove using its own capillary pressure. When the microchannel card is placed on a stage between a light source and a photodiode detector – a device that converts light into an electrical current – the device Lykotrafitis and Partola have developed measures how long it takes the blood to travel through the microchannel. A few minutes after the sample is placed on the microchannel, a digital screen displays a viscosity reading that indicates whether the patient is at elevated risk for cardiac events.
Once the test is completed, the used microchannel card is discarded and replaced with a new one. Since the device itself never comes in contact with the biological sample, practitioners don’t need to sterilize it in between patients or worry about cross-contamination.
Currently, to measure blood viscosity physicians would typically need to send large samples to an off-site lab for analysis in a rheometer, an instrument that measures viscosity mechanically. Commercial rheometers require large samples, take much longer, cost thousands of dollars, and are also commonly used to measure the viscosity of industrial liquids like oil, paint, or personal care products. The commercial equipment needs to be sterilized in between tests because of this multi-purpose capability. Travel time between the medical office where the blood was originally collected and the commercial facility where it is tested also means that samples are no longer reliable. This is all less than ideal for clinical applications.
In contrast, the device that Lykotrafitis and Partola are developing only requires a finger prick of blood, gives precise readings in just a few minutes, and will cost under a thousand dollars.
To commercialize their technology, the duo looked to Accelerate UConn, a growing entrepreneurial program that serves all UConn campuses. Accelerate UConn was launched in May 2015 and is the University’s National Science Foundation I-Corps site. The program teaches participants how to determine the market opportunity for their technology and who the most likely customers will be.
Helping scientists “get out of the lab” is one of the most important and challenging parts of the Accelerate UConn program, according to UConn vice president for research and former Accelerate UConn participant, Radenka Maric.
“There is a wealth of amazing ideas being developed at UConn and UConn Health every day, but to have an impact they need to reach beyond the lab” says Maric. “The Accelerate UConn program provides our world-class researchers with entrepreneurial tools to move these ideas closer to the market, where they can help our citizens, as well as our state economy.”
Partola served as the group’s entrepreneurial lead, which meant he was responsible for interviewing dozens of potential customers. He used the $3,000 award provided by Accelerate UConn to travel to Los Angeles, California, and speak with nurses, researchers, and pharmacists about his technology at the national conference of the Anticoagulation Forum.
Partola says the opportunity to speak with potential customers and the Accelerate UConn curriculum have had an impact on his outlook on entrepreneurship and being a scientist.
“It’s not just about having a technology that works and that you think meets a need,” he says. “Just because you build it doesn’t mean they’ll come. I learned that you need to find your customers first, and tailor a specific solution for their problem to be successful.”
The pair have formed a startup, Eir Medical Devices. They also recently completed the Connecticut Center for Entrepreneurship & Innovation (CCEI) Summer Fellowship Program, where they received a $15,000 stipend, intensive business support, and one-on-one coaching from industry experts. A panel of external judges were so impressed with the team’s technology and business plan that they have been named finalists in the upcoming Wolff New Venture Competition administered by CCEI.
In terms of advancing their research, Partola and Lykotrafitis are in early discussions with physicians at UConn Health and Yale University to conduct clinical trials.
To date, approximately 25 teams have successfully completed the Accelerate UConn program.
Applications are currently being accepted for the Fall 2017 cycle, which begins in October. The deadline to apply is Sept. 22, 2017. For more information and to access the application, go to www.accelerate.uconn.edu.
Lykotrafitis had previously conducted research that sparked the idea for this invention through support from the National Science Foundation (NSF-CMMI-1235025) and the American Heart Association (12SDG12050688). No resources from those previous awards were used to fund product development or testing of the current prototype device.